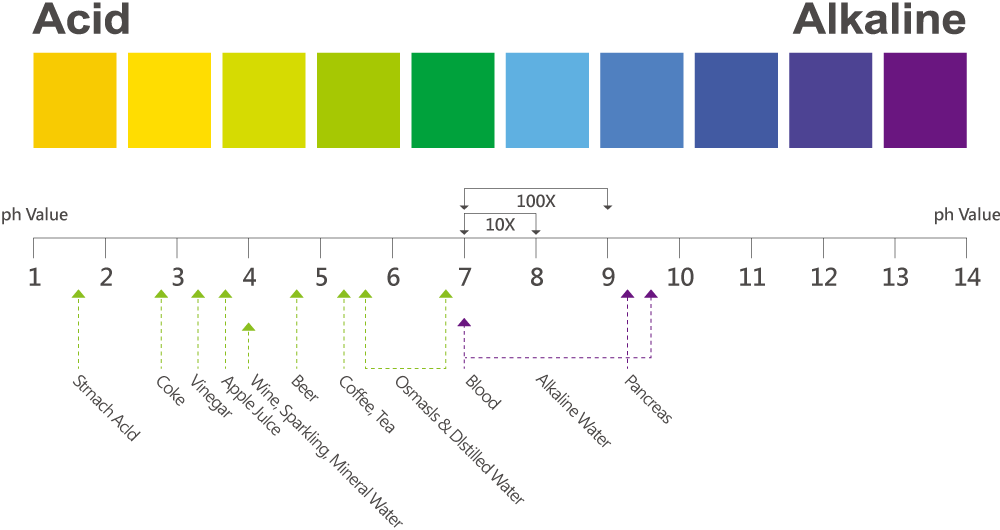
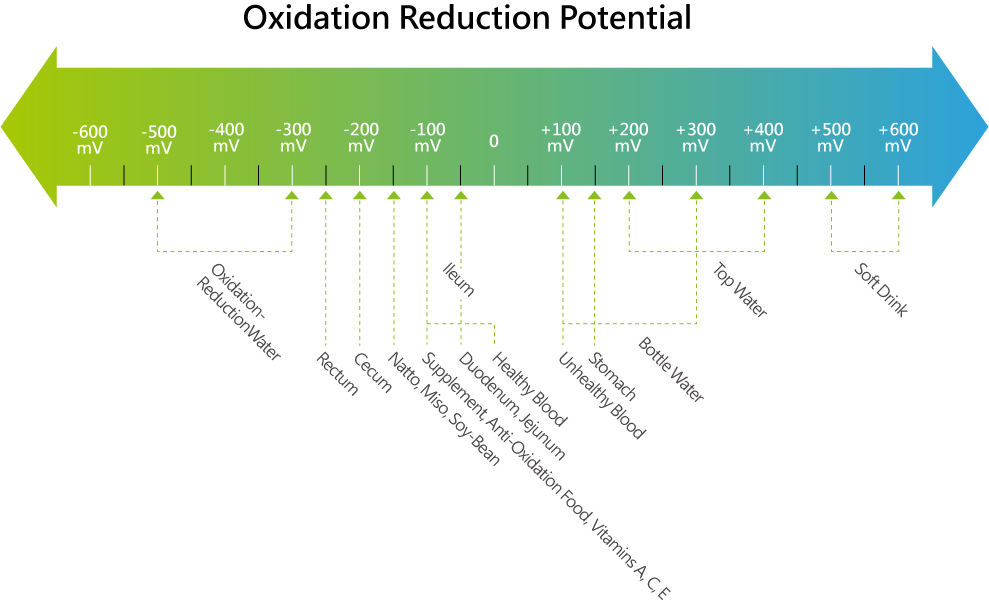
- pH (Potential Hydrogen)
- The pH value of an aqueous solution is measured on a scale of 0 to 14 with extreme acidity being 0 and extreme alkaline being 14. pH values represent the hydrogen ion (H+ and OH- ions) activity within the solution and reflect the tendency of hydrogen ions within the solution to interact with other components of the solution. Pure (neutral) water contains equal concentrations of H+ and OH- ions and has a pH around 7 at 25 °C (77 °F). This pH value varies with temperature. When an acid is dissolved in water, the water will contain a higher concentration of H+ ions and the pH will decrease to less than 7 (if at 25 °C (77 °F)). When an alkaline is dissolved in water, the water will contain a higher concentration of OH- ions and the pH will increase to more than 7 (if at 25 °C (77 °F)).
- ORP (Oxidation Reduction Potential or Redox Potential)
- ORP is a measure of the presence of oxidizing or reducing agents in a solution. During a reaction between components in a solution, there is a tendency to transfer electrons between the components. The component with a lower (more negative) ORP will have a tendency to lose an electron and is said to be "reduced". The component with a higher (more positive) ORP will have a tendency to gain electrons and is said to be "oxidized". ORP of a solution is measured in millivolts (mV) with a specialized electrode meter. The more negative the OPR reading, the greater the substances' tendency to give away electrons and be reduced; the more positive the ORP reading, the greater the substances' tendency to pick up electrons and be oxidized. Ionized water units simultaneously produce water with two levels of ORP, one with a high reduction potential → alkaline water; and one with a high oxidization potential → acidic water.